PCB Corrosion: Causes and How to Clean and Prevent.
jhdpcb@gmail.com
When it comes to printed circuit boards (PCBs), corrosion can be a nightmare. Not only can it cause serious damage to your PCBs, but it can also be a major safety hazard. As electronic devices become more complex and sophisticated, preventing corrosion is becoming increasingly important.
As a leading PCB manufacturer, JHDPCB understands the importance of preventing corrosion in printed circuit boards. We know that corrosion not only damages PCBs but can also pose a safety risk. In this article, we’ll take a deep dive into the best practices for PCB corrosion prevention, including tips and tricks for proper handling, storage, and maintenance. So whether you’re a seasoned engineer or a novice hobbyist, read on to learn how to keep your PCBs in top shape and avoid the headaches of corrosion.
What is PCB Corrosion?
Corrosion is the natural process of oxidation that occurs when metal is exposed to oxygen, causing it to rust and lose its valuable chemical properties. Printed circuit boards, which are primarily composed of metal, are susceptible to corrosion over time due to their exposure to oxygen.
As a result, it is important to take steps to prevent corrosion, such as using high-quality materials and implementing proper handling, storage, and maintenance practices. By doing so, PCB manufacturers can ensure that their products are resistant to corrosion in PCB and will perform reliably over the long term.
The corrosion reaction can be triggered by various pcb corrosion causes:
- Exposure to moisture and humidity;
- Exposure to harsh chemicals or contaminants;
- Presence of acidic or alkaline substances;
- High temperatures or thermal cycling;
- Poor quality materials or manufacturing processes;
- Electrochemical reactions with other noble metals or conductive materials;
- Poor design or layout of the PCB, leading to trapped moisture or contaminants;
- Inadequate or improper cleaning of the PCB before or after assembly;
- Improper storage or handling of the PCB, leading to exposure to moisture or contaminants.
Effects of PCB Corrosion.
PCB corrosion can have severe consequences on the functionality and reliability of electronic devices. Here are some of the issues that you can have:
- PCBs suffer from various types of corrosion including chemical, electrochemical, and galvanic corrosion. All forms of corrosion degrade the copper circuitry and lead to performance issues.
- As copper traces corrode, their conductivity decreases. This leads to higher electrical resistance which slows signal propagation and reduces the operating speed of the PCB. The impedance also changes which can interfere with signals. Click to learn more about the principle of PCB impedance control.
- Corroded copper traces become thinner and can even break completely. This causes intermittent or complete loss of electrical connections between components soldered to those traces. This results in components not powering on or malfunctioning.
- The insulating material surrounding traces – laminate and solder mask – also degrades with corrosion. This reduces the dielectric strength and insulation resistance of the PCB. Components may develop electrical shorts or leakage currents.
- Components soldered to corroded traces can become loose and contact reliability decreases. This eventually leads to unstable or unreliable electrical connections for those components.
- Severe corrosion can pit or hole the copper traces. This perforates the trace and forms an open circuit, completely disabling electrical connections through that trace. Learn more about PCB Traces.
- As corrosion progresses, electrical and mechanical integrity deteriorates. Performance becomes unpredictable and unreliable. Eventually the PCB may fail to function entirely.
- The effects of corrosion tend to worsen over time and accelerate as corrosion propagates across the PCB. Unless addressed, corrosion can render PCBs completely unusable.
What are the Types of Corrosion in Circuit Boards?
So, now let’s discuss the different types of PCB corrosion that can occur in circuit boards, including atmospheric, localized, galvanic, electrolytic, fretting, and intergranular corrosion. Understanding these corrosion types is crucial for the proper design, manufacturing, and maintenance of electronic devices.
General Attack Corrosion
There are multiple types of corrosion, but general attack corrosion is the most common. With general attack corrosion, oxygen and moisture in the air react with exposed copper surfaces. This reaction forms a copper oxide, which builds up over time and increases the electrical resistance of the copper traces.
As the copper oxide accumulates, it can exceed the tolerance thresholds for circuits and cause electrical failures. However, although the conductivity decreases, the bulk mechanical properties of the copper typically remain intact.
General corrosion affects the entire exposed copper surface more or less uniformly. This makes it easier to detect through visual inspection compared to localized corrosion, which impacts localized, isolated areas. Preventing general corrosion also tends to be simpler as it mainly requires enclosing the circuit board in a protective barrier like a conformal coating to block air and moisture.
Atmospheric Corrosion
Atmospheric corrosion is the most prevalent form of corrosion affecting printed circuit boards (PCBs). It occurs when moisture and oxygen in the atmosphere react with exposed metal traces on the PCB. All metals are susceptible to atmospheric corrosion to some degree. However, copper is particularly prone to it due to its high reactivity. Copper is often used for circuit traces, plated through holes, and other conductive elements in PCBs.
The atmospheric corrosion process starts when moisture accumulates on the exposed metal surface. Water molecules adsorb to the surface, forming a thin film. Oxygen then diffuses through this film and reacts with the copper metal. The corrosion reaction forms copper oxide, which has a lower electrical conductivity than pure copper. Over time, as more copper oxide forms, the electrical resistance of the copper traces increases. Once the resistance exceeds tolerance thresholds for a given circuit, electrical failures can result.
While atmospheric corrosion degrades the electrical performance of copper traces, their mechanical properties – such as hardness and tensile strength – are often still intact. This is because only a thin surface layer is typically affected by atmospheric corrosion. The bulk of the copper remains unchanged.
Localized Corrosion
Localized corrosion refers to corrosion that impacts limited, isolated areas of a copper circuit. There are 3 main types:
- Filiform corrosion: Filiform corrosion occurs when moisture penetrates beneath the surface finish and contacts exposed defects in the copper cladding. This establishes an electrolytic corrosion cell that allows an electric current to flow laterally. As the cell propagates, it consumes copper in its path, degrading large sections of circuitry over time.
- Crevice Corrosion: Crevice corrosion develops under components where residual contaminants trapped within crevices provide an electrolyte that supports a corrosion cell. Corrosion primarily attacks the base of the crevice where copper is exposed. As crevice corrosion progresses, it penetrates through copper traces, eventually severing them.
- Pitting Corrosion: Pitting corrosion forms small cavities or “pits” in the copper cladding due to localized galvanic corrosion cells. Copper is consumed at the pit base while hydroxyl ions accumulate within the pit, creating an acidic environment that accelerates corrosion. The pits grow both deeper and wider over time, eventually penetrating copper traces and causing electrical failures. However, corrosion deposits within the pits often obscure pitting corrosion, making it difficult to visually detect.
Galvanic Corrosion
Galvanic corrosion occurs when two dissimilar metals are in electrical contact within an electrolyte. In PCBs, this usually involves copper traces coupled with another metal component like gold or tin. When exposed to an electrolyte like moisture, an electrochemical cell is formed. Electrons flow from the more active metal (anode) to the less active metal (cathode), corroding the anode metal at an accelerated rate.
The key difference between galvanic and pitting corrosion is that galvanic corrosion requires two different metals in electrical contact, whereas pitting corrosion involves only one metal. Galvanic corrosion typically affects the entire interface between the two metals.
Electrolytic Dendrite Formation
When ionic contamination is present in moisture that contacts copper traces, dendritic growth can occur on the copper surfaces. Dendrites are branched, tree-like structures that form due to plating of copper ions from the electrolyte onto preferable crystallographic planes.
If adjacent copper traces grow dendrites that intersect, an electrical short circuit can result, potentially damaging circuitry. Dendrites are more likely to form where tracing is more dense and exposure to ionic contaminants is higher.
Intergranular Corrosion
Intergranular corrosion affects the grain boundaries within copper traces. Grain boundaries tend to have a higher concentration of impurities and defect sites, making them more susceptible to corrosive attack.
When chemicals are present that can penetrate grain boundaries, they can initiate corrosion reactions at the interface between adjacent copper grains. This corrosion then spreads along grain boundaries, potentially separating entire grains from the trace and compromising its integrity.
Corrosion from Flux Residue
Flux is essential during soldering to help the solder flow and adhere to metal surfaces. However, residue left from flux can be highly corrosive and cause problems over time.
Traditional fluxes contained chemicals like chlorine and other activated species that actively removed metal oxides during soldering. While effective, residues from these fluxes were highly corrosive and had to be thoroughly cleaned after soldering.
Modern no-clean fluxes use organic acids that decompose at soldering temperatures, leaving behind less corrosive residues. This is usually not an issue for reflow soldering where components experience high temperatures that fully decompose the flux. However, wave soldering may not reach temperatures high enough to fully decompose the flux, leaving acid residues behind. Click to read more detailed wave soldering and reflow soldering process.
Even small amounts of residual flux can cause corrosion problems over time as the acid residues absorb moisture from the environment. This forms an electrolyte that aids electrochemical corrosion reactions. The acids also lower the pH, further accelerating corrosion.
Fretting Corrosion
Fretting corrosion occurs due to small reciprocating motions between two contacting metal surfaces. In PCBs, this commonly happens with solder-plated switches as they open and close repeatedly during operation.
Each time the switch closes, the contact points rub against each other in a wiping action. This removes some of the thin oxide film that normally protects the metal surfaces from corrosion. When the oxide layer is breached, the exposed metal can then react with oxygen and moisture in the air to form corrosion products.
Electrolytic Corrosion
Electrolytic corrosion occurs when an electrolyte is present between two conductive traces that have a voltage potential. This allows ionic current to flow and metal ions to migrate, resulting in the plating or deposit of metallic dendrites that can cause short circuits.
For electrolytic corrosion to occur, three things are needed:
- 1) an electrolyte solution containing ions;
- 2) conductive traces that act as electrodes;
- 3) a voltage potential difference between the traces.
When these conditions exist, the conductive traces become the anode and cathode of an electrolytic cell. Ions in the electrolyte migrate toward the cathode trace, gaining electrons and plating out as tiny metal whiskers called dendrites.
The dendrites continue to grow as more metal ions plate out, driven by the electrical potential. Eventually, these dendritic projections from adjacent traces may contact each other and cause an unintended electrical short.
How to Remove Corrosion from PCB?
Here are the steps to remove corrosion from PCB (printed circuit board) in a professional manner:
- Step 1: Inspect the PCB for Corrosion Damage.
Look for green, blue, or white deposits on the copper traces of the PCB. These indicate the presence of copper oxide or other copper corrosion compounds. Note the location and extent of the corrosion. - Step 2:Clean the PCB Thoroughly:
Remove any debris, dirt, or other contaminants from the board using isopropyl alcohol and a soft-bristled brush. Flux residue or other chemicals on the board can interfere with the corrosion removal process. Handle the PCB with care to avoid damage. - Step 3: Determine If the Corrosion Can Be Removed Chemically:
For light to moderate corrosion, a chemical treatment may be attempted first before moving to more abrasive mechanical methods. Commercial PCB de-fluxing solutions containing ammonia or citric acid can dissolve copper oxide and other copper salts. Apply as directed and scrub with a soft brush. Repeat until the corrosion is removed. Rinse well with isopropyl alcohol and dry. - Step 4: Use Fine-Grit Abrasives for Stubborn Corrosion:
If chemical methods fail, more abrasive mechanical abrasion will be required. Use 400+ grit abrasive pads, fine-grade abrasive paper, or very fine-grade pumice to gently sand off the corrosion. Protect surrounding components from damage during sanding. Wipe away debris with isopropyl alcohol frequently. - Step 5: Protect the Cleaned Area:
Apply a thin coat of liquid solder mask or acrylic conformal coating to the cleaned copper areas. This prevents re-oxidation of the copper and protects it from future environmental damage. Allow it to cure as directed before handling the PCB further. - Step 6: Test the PCB for Proper Function:
If the corrosion was damaging or extensive, components or copper traces may need repair or replacement. Power up the PCB and test using diagnostic equipment to ensure proper function has been restored before putting the PCB back into service. Retreating or more advanced pcb corrosion repair may be required if issues are found.
Overall, carefully inspecting, cleaning, and determining the proper method for removing stubborn corrosion from a PCB, then protecting and testing the area to ensure full restoration can help extend the operational lifetime of printed circuit boards in a professional manner.
What are the Materials Useful for Cleaning Circuit Board Corrosion?
- Isopropyl Alcohol:
Isopropyl alcohol is very effective for cleaning corrosion on circuit boards. It is a mild solvent that can dissolve corrosion and oxidation, without damaging the circuit board components. Isopropyl alcohol comes in different concentrations, ranging from 70% to 99%. Higher concentrations are more effective at dissolving corrosion, but they also have a stronger solvent effect and may damage some components if not used properly. In most cases, a concentration of 91% is a good balance of dissolving power and safety. - Distilled Water:
Distilled water is a good choice for cleaning circuit boards because it does not contain ions or minerals that can cause corrosion. Regular tap water contains ions and contaminants that can damage sensitive electronic components. Distilled water provides a pure cleaning solution that will not degrade or leave behind residues on circuit traces. - Baking Soda:
Baking soda is a mild abrasive and alkalinizing agent that can help neutralize existing corrosion on circuit boards. Its abrasive qualities can also help remove light surface oxidation without damaging components. Baking soda raises the pH of the cleaning solution, which helps neutralize any acidic corrosion. Just be sure to thoroughly rinse the baking soda off after use. - Soft Bristle Brush:
If a specialized PCB brush is not available, a gentle paintbrush or toothbrush with soft bristles can be used. The soft bristles will flex and conform to component shapes without damaging them. A brush is useful for scrubbing away stubborn corrosion in hard-to-reach areas. Gentle brushing action is key to avoid scratching traces or loosening components. - Household Cleaners:
Photon-free household cleaners can help remove light corrosion, oils and other contaminants from circuit boards. Phosphates can actually accelerate corrosion over the long term so avoid cleaners containing them. Opt for mild, non-abrasive cleaners instead. Thoroughly rinse all residue from the board after cleaning. - Compressed Air:
Blowing compressed air through a circuit board is a non-chemical method of removing dust and debris. It can dislodge particles trapped between components or around chips. Take care when using compressed air, especially at high pressures, to avoid damaging delicate components. - Oven Drying:
Using an oven set to a low temperature of around 120 to 160 degrees Fahrenheit can help thoroughly dry a circuit board after cleaning. Place the board on a rack and allow at least 30 minutes to ensure all moisture has evaporated. Let the board cool completely before handling. - Lint-Free Towels:
Microfiber or other lint-free towels are ideal for drying circuit boards. They absorb moisture without leaving behind particles that can cause issues. Gently pat or blot the board surface to absorb excess water while minimizing abrasion. Inspect the towel frequently for trapped debris and switch to a clean section as needed. - Flux Removers and Solder Mask Cleaners:
Flux removers and solder masks are good to clean pcb corrosion. It contains strong solvents to dissolve built-up rosin flux, solder masks, and other contaminants from circuit boards. In small amounts, they can also dissolve some forms of light corrosion. However, the chemicals are quite strong, so they must be used carefully to avoid damaging components or lifting PCB traces. They should only be used according to the directions for the specific product.
Precautions When Cleaning PCB Corrosion:
Use caution when cleaning corroded PCBs. Aggressive cleaning methods can further damage the PCB and components. Here are some precautions to take.
- Ensure that the PCB is not connected to any power source, and remove any batteries before cleaning. If possible, remove sensitive components such as ICs, capacitors, and connectors that may be damaged during the cleaning process. Click to learn more about PCB components.
- Test the PCB first to determine the extent of the corrosion and damage. Components may already be dysfunctional due to corrosion PCB.
- Avoid abrasive cleaning methods like scrubbing or scraping that can remove copper traces or damage components. This includes steel wool, rough abrasive pads, and wire brushes.
- Gentle cleaning methods are better suited for PCBs. These include using chemical cleaners, corrosion removers, ultrasonic cleaning, and deionized water rinses.
- Apply chemical cleaners to the entire PCB, not just localized areas. This helps prevent barriers that can worsen corrosion later.
- Use corrosion removal chemicals specifically formulated for circuit boards. They tend to be milder and minimize damage to the PCB.
- Follow the manufacturer’s directions carefully when using any chemical cleaners. This includes handling precautions, application methods, and required safety equipment.
- Test the PCB after cleaning to confirm it is fully dry and that no residual moisture or chemicals remain. Residual liquids can later cause new corrosion issues. For more detailed JHD PCB inspection system, please click here.
- Inspect components for damage after cleaning. Gently reflow or reseat any loose components.
- Consider replacing heavily corroded components that are difficult to clean properly.
- Coat the PCB with a corrosion inhibitor or conformal coating after cleaning to help prevent future corrosion. This provides a barrier that most cleaners and contaminants cannot penetrate.
- Even after cleaning, corrosion tends to reappear over time. Frequent inspections and preventative maintenance are still required to sustainably combat PCB corrosion.
Cleaning Methods for Cleaning PCB Corrosion:
Now, here let’s talk about some cleaning methods for cleaning PCB corrosion.
- Solvent Cleaning:
Applying solvents like isopropyl alcohol or acetone to dissolve and flush away oils, fluxes and other organic residues. The solvents can carry corrosion products away as well. For light corrosion, a wipe with 90%+ isopropyl alcohol on a soft cloth may lift loose surface corrosion deposits. re-wipe with a clean cloth to remove any remaining residue. - Chemical Corrosion Removers:
Commercial refluxing solutions and PCB cleaners containing ammonia, citric acid, or hydrochloric acid will dissolve most copper corrosion compounds. Follow directions to scrub, rinse and neutralize the board. - Abrasive Pads and Sandpaper:
For heavy corrosion, very fine-grade abrasives (400+ grit) may be required to mechanically sand off corrosion. Use light pressure and wipe frequently with alcohol to prevent excess copper removal. - Ultrasonic Cleaning:
An ultrasonic part cleaner with an appropriate solvent can dislodge even stubborn corrosion from PCB surfaces. The solvent will depend on the types of components on the board. A multi-stage process including abrasives may be needed for severe corrosion. - Protective Coatings:
Apply a thin acrylic or solder mask coating to copper areas after cleaning to prevent re-oxidation. Cured coatings or epoxy coatings can also provide environmental protection for the PCB. Touch-up or re-coating may be required to maximize pcb corrosion protection.
How to Prevent PCB Corrosion?
To prevent printed circuit board (PCB) corrosion, several areas should be addressed:
Select the Appropriate Materials:
The choice of PCB substrate and finish plays a significant role in preventing corrosion. A substrate with a higher glass transition temperature (Tg) can withstand higher temperatures without losing its structural integrity, which in turn prevents the development of cracks and fissures that can lead to moisture ingress and corrosion.
Finishes such as Electroless Nickel Immersion Gold (ENIG) and Organic Solderability Preservative (OSP) provide better protection against environmental factors such as moisture, humidity, and chemical exposure. ENIG provides a thin layer of gold that resists oxidation, while OSP provides a thin layer of organic material that prevents oxidation.
Apply Conformal Coatings:
Conformal coatings are thin protective layers that are applied over the PCB to protect it against environmental factors. The choice of coating depends on the specific requirements of the PCB, such as its operating temperature range, flexibility, and resistance to chemicals.
Acrylic, silicone, polyurethane, and epoxy coatings are the most commonly used conformal coatings. Acrylic and silicone coatings are flexible and provide good moisture protection.
Polyurethane coatings provide excellent chemical resistance and are ideal for harsh environments. Epoxy resin coatings provide a hard and durable coating that is resistant to scratches and abrasions.
Control the Environment:
Controlling the environment in which the PCB is operated is critical for preventing moisture buildup and corrosion. The relative humidity should be maintained at a level between 30% and 60%.
Also, the temperature should be maintained within the operating range specified by the manufacturer. Air filtration can prevent the buildup of harmful chemicals and particulate matter that can cause corrosion.
Implement Proper Cleaning and Storage Procedures:
Proper handling and storage of PCBs can also help prevent corrosion. PCBs should be cleaned regularly using approved cleaning agents to remove any contaminants that can lead to corrosion.
The cleaning process should be followed by a thorough drying process to remove any residual moisture. PCBs should be stored in a dry and temperature-controlled environment to prevent moisture buildup and corrosion.
Galvanic Isolation:
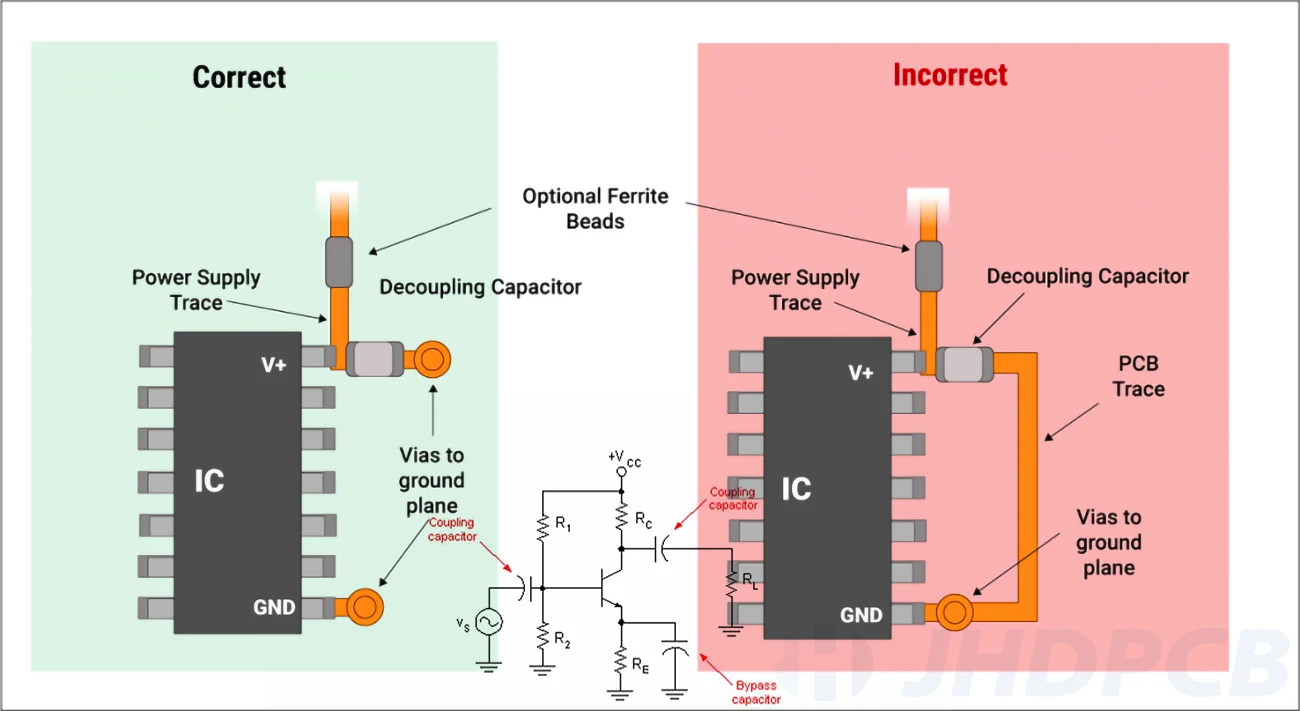
Preventing dissimilar base metals in contact with each other prevents galvanic corrosion from forming. Copper traces should not directly contact silver, brass, or stainless steel components or hardware. Nickel plating connector pins and using tin-lead solder also provide galvanic isolation from copper. Insulating washers should isolate board mounting points.
By following these best practices, you can significantly reduce the risk of corrosion and maintain the performance and functionality of electronic devices over time.
Work with a Reputable PCB Manufacturer:
Working with a reputable PCB manufacturer that understands the importance of corrosion prevention and uses high-quality materials and processes is critical for ensuring the reliability and longevity of electronic devices.
Reputable manufacturers use high-quality materials and processes that are designed to prevent corrosion. They also have strict quality control procedures in place to ensure that their products meet the highest standards of reliability and performance. JHDPCB is definitely your best choice, our strict quality control system and experienced team can avoid many problems in the manufacturing process and improve the quality of circuit boards.
By following these practices, you can significantly reduce the risk of corrosion and maintain the performance and functionality of your electronic devices over time. It is also important to work with a reputable PCB manufacturer that understands the importance of corrosion prevention and uses high-quality materials and processes.
At JHDPCB, we are committed to providing our customers with the highest quality PCBs that are designed to meet their specific needs and requirements. We use state-of-the-art manufacturing techniques and train a team of experienced professionals. Committed to ensuring the highest level of quality and reliability in PCBs, providing our customers with the best possible product foundation.
We take great pride in our work and are confident that our PCBs will exceed your expectations. Contact us today to learn more about our services and how we can help you with your next PCB project.